Atomic orbitals (AO). Quantum numbers
The wave function (7), which describes the state of the electron, is called atomic orbital(AO).
Quantum numbers. In quantum mechanics, each AO is defined by three quantum numbers.
Principal quantum number n. Can take integer values from 1 to ∞. The main quantum number determines:
energy level number;
energy range of electrons located at a given level;
orbital sizes;
the number of sublevels of a given energy level (the first level consists of one sublevel, the second - of two, the third - of three, etc.);
In the Periodic Table of Elements, the maximum value of the principal quantum number corresponds to the period number.
Orbital quantum number l.Determines the orbital angular momentum (momentum) of the electron, the exact value of its energy and the shape of the orbitals. Can take values 0, 1, 2, 3, …, ( n-1).
Atomic orbital– geometric image of the one-electron wave function ψ, which represents the region of the most probable presence of an electron in an atom. It limits the region of space in which the probability of finding an electron has a certain value (90 ... 99%). Sometimes an orbital is called the boundary surface of this region, and in drawings, as a rule, a cross section of this region is depicted by a plane passing through the origin of coordinates and lying in the plane of the drawing. The center of the atomic nucleus is placed at the origin. The concept of “orbital,” unlike “orbit,” does not imply knowledge of the exact coordinates of the electron. The orbital quantum number determines the shape of the atomic orbital. At l=0 is a sphere, with l=1 – volume eight (dumbbell), with l=2 – four-petal rosette.
Each value of the principal quantum number corresponds to n orbital quantum number values l(Table 1). For example, if n=1, then l takes only one value ( l=0), n=2 – two values: 0 and 1, etc. Each numerical value l a certain geometric shape of the orbitals corresponds and a letter designation is assigned. The first four letters of the designation are of historical origin and are associated with the nature of the spectral lines. s, p, d, f– the first letters of the English words used to name the spectral lines: sharp, principal, diffuse, fundamental. The symbols for other orbitals are given in alphabetical order: g, h, …
Table 1
Values of the principal and orbital quantum numbers
| Orbital quantum number l | Principal quantum number n | ||||||||||||||
| Meaning Letter designation | s | s | p | s | p | d | s | p | d | f | s | p | d | f | g |
The designation of any sublevel is determined by two quantum numbers - the main one (when writing, the numerical value is indicated) and orbital (when writing, the letter designation is indicated; orbital ()the numerical value is indicated by two quantum numbers - the main one). For example, the energy sublevel for which n=2 and l=1, should be designated as follows: 2p-sublevel. All orbitals with the same value l have the same geometric formula and, depending on the values of the principal quantum number, differ in size. For example, all orbitals for which l=0 (s-orbitals) are spherically symmetrical and differ in size depending on the value of the principal quantum number. The higher the value n, the larger the size of the orbitals.
Magnetic quantum number m l.Determines the possible values of the projection of the orbital angular momentum of an electron onto a fixed direction in space (for example, onto the axis z). It takes on negative and positive values l, including zero. Total number of values is 2 l+1:
The interaction of the magnetic field created by the electron with the external magnetic field depends on the value of the magnetic quantum number. If there is no external magnetic field, then the energy of the electron in the atom does not depend on m l. In this case, electrons with the same values n And l, but with different meanings m l have the same energy. If there is an external magnetic field, the energy of electrons with different m l varies.
In the general case, the magnetic quantum number characterizes the orientation of an AO in space relative to an external force. The magnetic quantum number determines the orientation of the orbital angular momentum relative to some fixed direction.
Total number of possible values m l corresponds to the number of ways to arrange the orbitals of a given sublevel in space, that is, the total number of orbitals at a given sublevel (Table 2).
table 2
Number of orbitals per sublevel
Orbital quantum number l=0 corresponds to the only value of the magnetic quantum number m l=0. These values l And m l characterize everything s-orbitals that have the shape of a sphere. Since in this case the magnetic quantum number takes only one value, each s-sublevel consists of only one orbital. Let's consider any R-sublevel. At l=1 orbitals have the shape of dumbbells (volume eights), the magnetic quantum number takes the following values: m l= -1, 0, +1. Hence, R-the sublevel consists of three AOs, which are located along the coordinate axes; they are designated p x, p y, p z accordingly (Fig. 1).
Rice. 1. Spatial form of s- and p-atomic orbitals.
For d-sublevel l=2, m l= -2, -1, 0, +1, +2 (total 5 values), and any d-sublevel consists of five atomic orbitals, which are located in a certain way in space (Fig. 2), and are designated accordingly.
Rice. 2. Spatial form of d-atomic orbitals.
Four out of five d- orbitals have the shape of four-lobed rosettes, each of which is formed by two dumbbells, the fifth AO is a dumbbell with a torus in the equatorial plane (-orbital) and is located along the axis z. The orbital lobes are located along the x and y axes. The orbital lobes are located symmetrically between the corresponding axes.
The fourth energy level consists of four sublevels - s, p, d And f. The first three of them are similar to those described above, and the fourth f-the sublevel consists of seven AOs, the spatial form of which is quite complex and is not discussed in this section.
S. Goudsmit and J. Uhlenbeck, to describe some subtle effects in the spectrum of the hydrogen atom in 1925, hypothesized the presence of the electron’s own angular momentum, which they called spin. Spin cannot be expressed in terms of coordinates and momenta; it has no analogue in classical mechanics. Spin number s electron takes only one value equal to the projection of the spin vector onto a certain direction of the external field (for example, onto the axis z) is determined spin quantum numberm S , which can take two values: m S =
The concept of “spin” was introduced to characterize a specific quantum property of an electron. Spin is a manifestation of relativistic effects at the microscopic level.
The electron has four degrees of freedom. The spin quantum number takes only discrete values: Thus, the state of an electron in an atom is determined by a set of values of four quantum numbers: n, l, m l, m S.
Designation and structure of electronic energy levels. Let's define some terms that are used to explain the physical meaning of quantum numbers. A group of orbitals having the same orbital quantum number forms energy sublevel. The set of all orbitals with the same value of the principal quantum number forms energy level.
The structure of atomic electronic levels can be depicted in two ways: in the form of electronic formulas and electron diffraction diagrams. When writing electronic formulas, two quantum numbers n and l are used: the first level is 1 s; second – 2 s, 2p; third – 3 s, 3p, 3d; fourth – 4 s, 4p, 4d, 4f etc. (Table 3).
Table 3
Structure of electronic energy levels of an atom
The structure of electronic levels is more fully described using three quantum numbers: n, l, m l. Each JSC is conventionally depicted in the form of quantum cells, next to which a level number and a sublevel symbol are placed.
The electron has a dual nature: in different experiments it can exhibit the properties of a particle and a wave. Properties of the electron as a particle: mass, charge; wave properties- in the features of motion, interference and diffraction.
The movement of an electron obeys the laws quantum mechanics .
The main characteristics that determine the movement of an electron around the nucleus: energy and spatial features of the corresponding orbital.
When interacting (overlapping) atomic orbitals(JSC ) belonging to two or more atoms are formed molecular orbitals(MO).
Molecular orbitals are filled with shared electrons and carry out covalent bond.
Before the formation of molecular orbitals, there may be hybridization of atomic orbitals of one atom.
Hybridization – changing the shape of some orbitals during the formation of a covalent bond to more effectively overlap them. Identical hybrids are formed JSC who participate in education MO, overlapping the atomic orbitals of other atoms. Hybridization is possible only for atoms that form chemical bonds, but not for free atoms.

Hydrocarbons
Main questions:
- Hydrocarbons. Classification. Nomenclature.
- Structure. Properties.
- Application of hydrocarbons.
Hydrocarbons- a class of organic compounds that consist of two elements: carbon and hydrogen.


Select isomers and homologues:

Name the alkanes:
 ____________________________________________
____________________________________________
 __________________________________________
__________________________________________

Ä nitration reaction (Konovalov reaction, 1889) is the reaction of hydrogen substitution with a nitro group.
Conditions: 13% HNO 3, t = 130 – 140 0 C, P = 15 – 10 5 Pa. On an industrial scale, nitration of alkanes is carried out in the gas phase at 150 – 170 0 C with nitrogen oxide (IV) or nitric acid vapor.
CH 4 + HO – NO 2 → CH 3 – NO 2 + H 2 O
nitromethane
@ Solve tasks:
1. The composition of alkanes is reflected by the general formula:
a) C n H 2 n +2; b) C n H 2 n -2; c) C n H 2 n; d) C n H 2 n -6 .
2. What reagents can alkanes react with:
A) Br 2 (solution); b) Br 2, t 0; V) H 2 SO 4; G) HNO 3 (diluted), t 0 ; d) KMnO 4 ; e) CON?
Answers: 1) reagents a, b, d, d; 2) reagents b, c, f;
3) reagents b, d; 4) reagents b, d, d, f.
- Establish a correspondence between the type of reaction and the reaction scheme (equation):
- Indicate the substance that is formed during complete chlorination of methane:
a) trichloromethane; b) carbon tetrachloride; c) dichloromethane; d) tetrachloroethane.
- Specify the most probable product of monobromination of 2,2,3-trimethylbutane:
a) 2-bromo-2,3,3-trimethylbutane; b) 1-bromo-2,2,3-trimethylbutane;
c) 1-bromo-2,3,3-trimethylbutane; d) 2-bromo-2,2,3-trimethylbutane.
Write an equation for the reaction.
Wurtz reaction – effect of metallic sodium on halogen derivatives of hydrocarbons. When two different halogen derivatives react, a mixture of hydrocarbons is formed, which can be separated by distillation.
| CH 3 I + 2 Na + CH 3 I → C 2 H 6 + 2 NaI |
@ Solve tasks:
1. Indicate the name of the hydrocarbon that is formed when bromoethane is heated with sodium metal:
a) propane; b) butane; c) pentane; d) hexane; e) heptane.
Write an equation for the reaction.
- What hydrocarbons are formed when metallic sodium acts on the mixture:
a) iodomethane and 1-bromo-2-methylpropane; b) 2-bromopropane and 2-bromobutane?
Cycloalkanes
1. For small cycles (C 3 – C 4) are characteristic addition reactions hydrogen, halogens and hydrogen halides. The reactions are accompanied by the opening of the cycle.
2. For other cycles (From 5 and above) typical substitution reactions.

Unsaturated hydrocarbons(unsaturated):
| Alkenes (olefins, unsaturated hydrocarbons with a double bond, ethylene hydrocarbons): Structure: sp 2 -hybridization, planar arrangement of orbitals (flat square). Reactions: addition (hydrogenation, halogenation, hydrohalogenation, polymerization), substitution (not typical), oxidation (combustion, KMnO 4), decomposition (without oxygen access). |  |
 |  |
 |  |
@ Solve tasks:
- What is the hybridization of carbon atoms in an alkene molecule:
![]()
a) 1 and 4 – sp 2, 2 and 3 – sp 3; b) 1 and 4 – sp 3, 2 and 3 – sp 2;
c) 1 and 4 – sp 3, 2 and 3 – sp; d) 1 and 4 – not hybridized, 2 and 3 – sp 2 .
2. Name the alkene:


- Draw up reaction equations using 1-butene as an example, and name the resulting products.

4. In the transformation scheme below, ethylene is formed in the reaction:
a) 1 and 2; b) 1 and 3; c) 2 and 3;
d) ethylene is not formed in any reaction.
- Which reaction goes against Markovnikov's rule:
a) CH 3 – CH = CH 2 + HBr →; b) CH 3 – CH = CH 2 + H 2 O →;;
c) CH 3 – CH = CH – CH 2 + HCI →; d) CCI 3 – CH = CH 2 + HCI →?

þ Dienes with conjugated bonds:hydrolysis 1,3-butadiene – 2-butene is formed (1,4-addition):
þ hydrogenation 1,3-butadiene in the presence of a catalyst Ni - butane:
þ halogenation 1,3-butadiene – 1,4-addition (1,4 – dibromo-2-butene):
þ polymerization of dienes:


Polyenes(unsaturated hydrocarbons with many double bonds) are hydrocarbons whose molecules contain at least three double bonds.
Preparation of dienes:
Ø effect of alcohol solution of alkali:
Ø Lebedev's method (divinyl synthesis):
Ø dehydration of glycols (alkanediols):
| Alkynes (acetylenic hydrocarbons, hydrocarbons with one triple bond): Structure: sp hybridization, linear arrangement of orbitals. Reactions: addition (hydrogenation, halogenation, hydrohalogenation, polymerization), substitution (formation of salts), oxidation (combustion, KMnO 4), decomposition (without access of oxygen). |  5-methylhexine-2
1-pentine 3-methylbutine-1 5-methylhexine-2
1-pentine 3-methylbutine-1
|
|||
 | 1. Which hydrocarbons correspond to the general formula C n H 2n-2: a) acetylene, diene; | |||
| b) ethylene, diene; | ||||
| c) cycloalkanes, alkenes; d) acetylene, aromatic? 2. A triple bond is a combination of: a) threeσ bonds; | ||||
| c) cycloalkanes, alkenes; b) one σ-bond and two π-bonds; c) two σ-bonds and one π-bond; d) threeπ bonds.). | ||||
| c) cycloalkanes, alkenes; 3. Create the formula for 3-methylpentine -3. | ||||
| I. Addition reactions v | ||||
| c) cycloalkanes, alkenes; Hydrogenation occurs through the stage of formation of alkenes: | ||||
| Addition of halogens | ||||
| occurs worse than in alkenes: Alkynes discolor bromine water ( | ||||
| ð qualitative reaction Addition of hydrogen halides: | ||||
| ð The addition products to unsymmetrical alkynes are determined: | ||||
| Markovnikov's rule: Adding water (hydration) | – reaction of M.G. Kucherov, 1881. For acetylene homologues, the product of addition of water is a ketone: | |||
| III. Formation of salts (acid properties) – substitution reactions | ||||
| Interaction with active metals: Acetylenides are used for the synthesis of homologues. Interaction of alkynes with ammonia solutions of silver oxide or copper(I) chloride): | Qualitative reaction to the final triple bond - the formation of a grayish-white precipitate of silver acetylide or red-brown copper (I) acetylide:. | |||
HC ≡ CH + CuCI → CuC ≡ CCu ↓ + 2HCI
s-Orbitals, as shown above, have a spherical shape and, therefore, the same electron density in the direction of each three-dimensional coordinate axis:
At the first electronic level of each atom there is only one s- orbital. Starting from the second electronic level in addition to s- three orbitals also appear R-orbitals. They are shaped like three-dimensional eights, this is what the area of the most likely location looks like R-electron in the region of the atomic nucleus. Each R-the orbital is located along one of three mutually perpendicular axes, in accordance with this in the name R-orbitals indicate, using the corresponding index, the axis along which its maximum electron density is located:
In modern chemistry, an orbital is a defining concept that allows us to consider the processes of formation of chemical bonds and analyze their properties, while attention is focused on the orbitals of those electrons that participate in the formation of chemical bonds, that is, valence electrons, usually the electrons of the last level.
The carbon atom in the initial state has two electrons in the second (last) electronic level. s-orbitals (marked in blue) and one electron in two R-orbitals (marked in red and yellow), the third orbital is p z-vacant:
Hybridization.
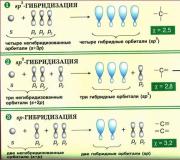
In the case when a carbon atom participates in the formation of saturated compounds (not containing multiple bonds), one s- orbital and three R-orbitals combine to form new orbitals that are hybrids of the original orbitals (the process is called hybridization). The number of hybrid orbitals is always equal to the number of original ones, in this case, four. The resulting hybrid orbitals are identical in shape and outwardly resemble asymmetrical three-dimensional figure eights:
The whole structure appears to be inscribed in a regular tetrahedron - a prism assembled from regular triangles. In this case, the hybrid orbitals are located along the axes of such a tetrahedron, the angle between any two axes is 109°. Carbon's four valence electrons are located in these hybrid orbitals:
Participation of orbitals in the formation of simple chemical bonds.
The properties of electrons located in four identical orbitals are equivalent; accordingly, the chemical bonds formed with the participation of these electrons when interacting with atoms of the same type will be equivalent.
The interaction of a carbon atom with four hydrogen atoms is accompanied by the mutual overlap of elongated hybrid orbitals of carbon with spherical orbitals of hydrogen. Each orbital contains one electron; as a result of overlap, each pair of electrons begins to move along the united molecular orbital.
Hybridization only leads to a change in the shape of the orbitals within one atom, and the overlap of the orbitals of two atoms (hybrid or ordinary) leads to the formation of a chemical bond between them. In this case ( cm. Figure below) the maximum electron density is located along the line connecting two atoms. Such a connection is called an s-connection.
Traditional writing of the structure of the resulting methane uses the valence bar symbol instead of overlapping orbitals. For a three-dimensional image of a structure, the valence directed from the drawing plane to the viewer is shown in the form of a solid wedge-shaped line, and the valence extending beyond the drawing plane is shown in the form of a dashed wedge-shaped line:
Thus, the structure of the methane molecule is determined by the geometry of the hybrid orbitals of carbon:
The formation of an ethane molecule is similar to the process shown above, the difference is that when the hybrid orbitals of two carbon atoms overlap, a C-C bond is formed:
The geometry of the ethane molecule resembles methane, bond angles are 109°, which is determined by the spatial arrangement of carbon hybrid orbitals:
Participation of orbitals in the formation of multiple chemical bonds.
The ethylene molecule is also formed with the participation of hybrid orbitals, but only one is involved in hybridization s-orbital and only two R-orbitals ( p x And RU), third orbital – p z, directed along the axis z, does not participate in the formation of hybrids. From the initial three orbitals, three hybrid orbitals arise, which are located in the same plane, forming a three-rayed star, the angles between the axes are 120°:
Two carbon atoms attach four hydrogen atoms and also connect to each other, forming a C-C s-bond:
Two orbitals p z, which did not participate in hybridization, overlap each other, their geometry is such that the overlap occurs not along the C-C communication line, but above and below it. As a result, two regions with increased electron density are formed, where two electrons (marked in blue and red) are located, participating in the formation of this bond. Thus, one molecular orbital is formed, consisting of two regions separated in space. A bond in which the maximum electron density is located outside the line connecting two atoms is called a p-bond:
The second valence feature in the designation of a double bond, which has been widely used to depict unsaturated compounds for centuries, in the modern understanding implies the presence of two regions with increased electron density located on opposite sides of the C-C bond line.
The structure of the ethylene molecule is determined by the geometry of hybrid orbitals, the H-C-H bond angle is 120°:
During the formation of acetylene, one s-orbital and one p x-orbital (orbitals p y And p z, do not participate in the formation of hybrids). The two resulting hybrid orbitals are located on the same line, along the axis X:
The overlap of hybrid orbitals with each other and with the orbitals of hydrogen atoms leads to the formation of C-C and C-H s-bonds, represented by a simple valence line:
Two pairs of remaining orbitals p y And p z overlap. In the figure below, colored arrows show that, from purely spatial considerations, the most likely overlap of orbitals with the same indices x-x And ooh. As a result, two p-bonds are formed surrounding a simple s-bond C-C:
As a result, the acetylene molecule has a rod-shaped shape:
In benzene, the molecular backbone is assembled from carbon atoms having hybrid orbitals composed of one s- and two R-orbitals arranged in the shape of a three-rayed star (like ethylene), R-orbitals not involved in hybridization are shown semi-transparent:
Vacant orbitals, that is, those not containing electrons (), can also participate in the formation of chemical bonds.
High level orbitals.
Starting from the fourth electronic level, atoms have five d-orbitals, their filling with electrons occurs in transition elements, starting with scandium. Four d-orbitals have the shape of three-dimensional quatrefoils, sometimes called “clover leaves”, they differ only in orientation in space, the fifth d-orbital is a three-dimensional figure eight threaded into a ring:
d-Orbitals can form hybrids with s- And p- orbitals. Options d-orbitals are usually used in the analysis of the structure and spectral properties of transition metal complexes.
Starting from the sixth electronic level, atoms have seven f-orbitals, their filling with electrons occurs in the atoms of lanthanides and actinides. f-Orbitals have a rather complex configuration; the figure below shows the shape of three of seven such orbitals, which have the same shape and are oriented in space in different ways:
f-Orbitals are very rarely used when discussing the properties of various compounds, since the electrons located on them practically do not take part in chemical transformations.
Prospects.
At the eighth electronic level there are nine g-orbitals. Elements containing electrons in these orbitals should appear in the eighth period, while they are not available (element No. 118, the last element of the seventh period of the Periodic Table, is expected to be obtained in the near future; its synthesis is carried out at the Joint Institute for Nuclear Research in Dubna).
Form g-orbitals, calculated by quantum chemistry methods, are even more complex than those of f-orbitals, the region of the most probable location of the electron in this case looks very bizarre. Below is the appearance of one of nine such orbitals:
In modern chemistry, concepts of atomic and molecular orbitals are widely used in describing the structure and reaction properties of compounds, also in analyzing the spectra of various molecules, and in some cases to predict the possibility of reactions occurring.
Mikhail Levitsky
ORBITAL
ORBITAL, in ELEMENTARY PARTICLE PHYSICS - the surface of space around the atomic NUCLEUS in which ELECTRONS can move. There is a high probability of the presence of an electron in such an orbital. It may contain one or two electrons. The orbital has a shape and energy corresponding to the QUANTUM NUMBER of the atom. In molecules, bond electrons move in the combined electric field of all nuclei. In this case, atomic orbitals become molecular orbitals, regions that surround two nuclei that have a characteristic energy and contain two electrons. These molecular orbitals, formed from atomic orbitals, constitute CHEMICAL BONDS.
Atomic orbitals describe the surface around the nucleus of an atom, which most likely contains electrons.
They can also be called "energy clouds". Their existence explains chemical bonds. Electrons are contained within atomic or molecular structures arranged into energy levels. The first level is characterized by only one type of electron: it has one s-orbital (A), shown relative to the x, y and z axes of the atom. The maximum number of electrons that can be in this energy level is two. For the second type of electrons, the orbital has the shape of two connected spheres located symmetrically relative to the nucleus. Such an orbital is called a p-orbital (B) V atom three such orbitals, and they are located at right angles to each other (1,2, 3) Orbitals that have regular spherical shapes are conventionally designated as pear-shaped clouds for clarity of the picture . In addition, there are also five d-orbitals (C-G), each of which consists of four pear-shaped lobes on two perpendicular axes, intersecting at the G nucleus - a combination of two p-orbitals..
Scientific and technical encyclopedic dictionary
See what "ORBITAL" is in other dictionaries:
Orbital: Atomic orbital. Molecular orbital. A list of meanings of a word or phrase with links to relevant articles. If you came here from... Wikipedia orbital - is the complete set of wave functions of an electron located in the field of nuclides and the averaged field of all other electrons interacting with the same nuclides. Atomic orbital is the allowed state of an electron in an atom, a geometric image,... ...
Chemical terms A function of spatial variables of one electron, which has the meaning of a wave function of an electron located in the field of an atomic or molecular core. If such a function takes into account the spin electron, then it is called. spin O. For more details, see Molecular orbital... ...
Orbital: Atomic orbital. Molecular orbital. A list of meanings of a word or phrase with links to relevant articles. If you came here from... Wikipedia Physical encyclopedia - orbitale. physical Atomic and molecular wave functions of an electron located in the field of one or more atomic nuclei and in the average field of all other electrons of the atom or molecule in question. NES 2000…
Historical Dictionary of Gallicisms of the Russian Language - (from Lat. orbita path, track), wave function describing the state of one electron in an atom, molecule or other quantum system. In the general case, quantum chemistry. the term O. is used for any function that depends on the variables x, y, z of one... ...
Orbital: Atomic orbital. Molecular orbital. A list of meanings of a word or phrase with links to relevant articles. If you came here from... Wikipedia- orbitalė statusas T sritis chemija apibrėžtis Banginė funkcija, apibūdinanti elektrono judėjimą atome arba molekulėje; erdvė, kurioje elektrono buvimas labiausiai tikėtinas. atitikmenys: engl. orbital rus. orbital... Chemijos terminų aiškinamasis žodynas
Orbital: Atomic orbital. Molecular orbital. A list of meanings of a word or phrase with links to relevant articles. If you came here from... Wikipedia- orbitalė statusas T sritis fizika atitikmenys: engl. orbital vok. Orbital, n rus. orbital, f pranc. orbitale, f … Fizikos terminų žodynas
Orbital: Atomic orbital. Molecular orbital. A list of meanings of a word or phrase with links to relevant articles. If you came here from... Wikipedia- orbit al, and... Russian spelling dictionary
Orbital: Atomic orbital. Molecular orbital. A list of meanings of a word or phrase with links to relevant articles. If you came here from... Wikipedia- With. Orbit buencha bashkaryl torgan. Orbit buencha hәrәkәt itә torgan yaki shunyn өchen bilgelәngәn… Tatar telen anlatmaly suzlege
Orbital: Atomic orbital. Molecular orbital. A list of meanings of a word or phrase with links to relevant articles. If you came here from... Wikipedia- A function of spatial variables of one electron, which has the meaning of the wave function of an individual electron in the field of the effective atomic or molecular core ... Polytechnic terminological explanatory dictionary
Books
- Dictionary of Cosmic Philosophy, Seklitova L.A. , This dictionary includes words and concepts most often found in esoteric literature. The need to compile it is dictated by the fact that many well-known... Category: Health and personal development Series: Beyond the Unknown Publisher: Amrita-Rus,
- Dictionary of Cosmic Philosophy, Seklitova Larisa Aleksandrovna, Strelnikova Lyudmila, This dictionary includes words and concepts that are most often found in esoteric literature. The need to compile it is dictated by the fact that many well-known... Category:
Beauty hidden from us
To each their own it is beautiful.
Cicero
Who are “we” and what are they hiding from us? We will talk about chemists, however, this applies to everyone. On our journey we will need a guidebook, or a map of the area, in order to quickly reach the hidden goal. A chemist always has such a guide at hand - this is the periodic table. The further story will become much more interesting if you have this table at hand.
Imagine that you have met an interesting person from whom you constantly learn unusual and interesting information.
In our article, electrons provide information. It is they (primarily valence electrons) that determine the behavior of substances formed by chemical elements and the endless variety of their chemical transformations. Let's consider the conditions under which electrons live. It cannot be said that someone is hiding the architecture of their home from us, but few people know the true picture.
Nature is an excellent designer
Recall that the region of space occupied by an electron in an atom or molecule is called an orbital. Not only the concept of orbitals itself has become familiar and even popular, but also their appearance, which can sometimes be seen on book covers. For example, on the cover of one of the school chemistry textbooks there is a diagram of a water molecule, and a similar plot is a diagram of a methane molecule (Fig. 1).
Both designs are very attractive.
The orbitals located inside the tetrahedron, resembling elongated balloons, are in contact with the spherical orbitals.
In the methane molecule there are molecular orbitals, but we will focus on simpler objects - atomic orbitals. Where are electrons located in isolated atoms that are not connected by chemical bonds? Having admired the pictures shown, let’s put emotions aside and add a sad note - the true molecular orbitals in methane are quite noticeably different in appearance from what is shown in most pictures. We'll talk about why this happened a little later.
What are they really like? So, an electron moves in an atom around the nucleus not along a fixed line - an orbit, but occupies a certain region of space. Previously they used the term “orbit”, but gradually came to the idea that orbit (from lat. orbita
There is a region of space where the electron is most likely to be found. For purposes of clarity, the orbital is limited to a surface that outlines the region of space where the probability of an electron appearing is greatest, in other words, where the electron density is maximum (Fig. 2b). So, the orbital should be perceived as a kind of volumetric body, inside of which the electron is located with a probability of 95%.
The electron orbital of the hydrogen atom has a spherical (spherical) shape, therefore, the electron density in the direction of each axis of three-dimensional coordinates is the same (Fig. 3). This is the so-called s-orbital.
To date, five types of orbitals have been described: s, p, d, f And g. The names of the first three were formed historically, then the alphabetic principle was chosen, so these letters do not carry any hidden meaning.
Orbitals exist regardless of whether they contain electrons (occupied orbitals) or are absent (vacant orbitals). It is interesting that the atom of each element, starting with hydrogen and ending with the last element obtained today, has a complete set of all orbitals at all energy levels, and their filling with electrons occurs as the atomic number of the element increases, i.e. charge of the nucleus of an atom. s The atom of every chemical element contains -orbitals, with one such at each energy level s orbitals.
They all have a spherical shape, but this is where Nature has prepared a surprise. If at the first energy level s-orbital is a solid body, then on the second it is a sphere within a sphere, and on the third there are three spheres nested one inside the other (Fig. 4).
By the way, the third energy level begins to be filled in the elements of the third period of the periodic system (the second level - in the elements of the second period, the fourth level - in the elements of the fourth period, etc.). Thus, Nature encrypted the same information twice - in the numbers of periods and in the number of layers in s-orbitals.
Besides s- orbitals also exist R-orbitals. Three such orbitals first appear at the second energy level. At each subsequent level there are also always three of them. Whatever they called R X, -orbitals - both with two-blade propellers and dumbbells; Now the name “volumetric eights” has been established. All three orbitals are identical in appearance, but are oriented differently in space. Their maximum electron density is concentrated along one of three coordinate axes - y z or R-orbitals.
(Fig. 5).
This is exactly what the region of the most probable location of an electron looks like when it settles on R These orbitals are depicted in this way in all textbooks. It is interesting that the true form of these orbitals (Fig. 6) differs markedly from the generally accepted one (see Fig. 5). R They do not look like elongated drops at all, but rather resemble buns or buttons. R It is in these orbitals that electrons are located in the elements of the second period of the periodic table, starting with boron and ending with neon. R It is quite logical that these elements are called R-elements. R Usually in D.I. Mendeleev’s table
-elements are highlighted with a special color. At the third energy level there is also
-orbitals, but they are somewhat different in appearance from their “relatives” living on the “second floor” (Fig. 7). U 3 s-orbitals a “skirt” appears, the whole design is similar to an antique table lamp, only seemingly double. These orbitals are gradually filled with electrons from aluminum to argon, they are also called R-elements. In the periodic table they have exactly the same color as
Why are they so distorted? R-orbitals depicted in books? There is no malicious intent here, it is the result of simplification. In order to explain the interactions that occur, it is quite enough to indicate the spatial location of the orbitals and their approximate outlines. In addition, the drop-shaped shape is much easier to depict and with its help it is more convenient to convey the overlap of orbitals that occurs during the formation of chemical bonds. Let's take an example closer to us. When we write a reaction equation, we represent the atoms using chemical element symbols. At the same time, we do not depict all the electrons near each of them and do not indicate which of the electrons R, Which one then - s.
In most cases this is not required. If such a need arises, then, for example, a pair of electrons is introduced into the reaction scheme to carry out a covalent bond.
However, the true shapes of the orbitals are important and are taken into account in complex calculations that take into account the spatial interactions of the orbitals.
Only rare enthusiasts take on this difficult work. Thanks to their efforts, we can see how everything really looks, and at the same time appreciate the bizarre fantasy of Nature.
Everyone prefers their own orbitals R If the form d-orbitals are most often discussed in organic chemistry textbooks, then the following d-orbitals are a favorite topic in coordination chemistry, which examines the properties of complex compounds. These orbitals appear at the third energy level. At this and every subsequent level there are always five of them.
d-Orbitals begin to be populated by electrons from elements of the fourth period, the so-called transition elements (more often called d-elements), starting with scandium and ending with zinc. In the periodic table s-elements are painted in a color different from R- And d-elements. R Form d-orbitals are somewhat more complex than those of d-orbitals.
Four d-orbitals are usually depicted in this way, regardless of what level they belong to. The most interesting thing is that shown in Fig. 9, the image is almost no different from the true one, but this applies only to the third level orbitals (Fig. 10).
In the fifth period, filling occurs d-orbitals of the fourth energy level, as a result new ones appear d-elements, from yttrium to cadmium, in the table they are colored exactly the same as d-elements of the previous period. The entire previous story has prepared us for the fact that appearance 4 d-orbitals will be slightly different than 3 d-orbitals. This is actually the case (Fig. 11). The drop-shaped shape gives way to a mushroom-shaped one, and something like additional legs appears. For similar 5 d-orbitals begin to settle electrons in d-elements of the sixth period, i.e. in lanthanum and further from hafnium to mercury.
Now it no longer seems surprising that d-orbitals of the fifth energy level have an even more complex shape (Fig. 12).
If only a simplified image of them and a purely qualitative discussion of the form is required, then we can conditionally assume that all considered d-orbitals have the shape shown in Fig. 10. We have a pleasant opportunity to see what everything really looks like, thanks to the efforts of a scientist from the University of Sheffield, Mark Winter.
Not everyone saw it
At the fourth energy level seven appear f- orbitals, and at each subsequent level there are always seven of them. They begin to be populated by electrons from elements called lanthanides (also called f-elements), starting with cerium and ending with lutetium. Their cells in the periodic table are also painted in a special color. If all the previously mentioned orbitals can be seen in one form or another in various books, then the appearance f- Few people are familiar with orbitals.
Meanwhile, purely externally, they fully deserve to not only appear on the pages of the book, but also decorate the cover, however, judge for yourself (Fig. 13). f In the next period of the periodic table, new ones naturally appear. f--elements, from thorium to lawrencium, have the form
orbitals is even more unusual; a reduced ring appears between two large tori (donuts) (Fig. 14).
It would seem that the spatial fantasy of Nature should be exhausted, but then even more sophisticated designs await us.
The ultimate fantasy of Nature f- Behind orbitals follow nine orbitals. orbitals follow nine They appear at the next (fifth) energy level, i.e. in full accordance with the established order - each new level carries with it a new type of orbitals. orbitals follow nine It was said earlier that every atom has a complete set of all orbitals, starting with hydrogen. However, in order for an electron to settle in a certain upper orbital, all previous orbitals must be filled (for more details, see: Chemistry, 2000, No. 22. Chemical elements. Achievements and prospects). We cannot yet name those elements that contain electrons on orbitals follow nine orbitals, such elements have not yet been obtained. Calculations have shown that for the first time an electron can be placed in this orbital at chemical element No. 125. However, the wait is most likely not so long; element No. 118 has already been obtained today. The series will begin with element No. 125 orbitals follow nine elements (each subsequent one will add one electron per
orbitals), these elements will be fundamentally new; they have no analogues in the entire previous periodic table. They are not so easy to obtain, but it will be even more difficult to study their properties, since they will most likely be short-lived radioactive elements. Without waiting for the moment when they are received, we can already admire the appearance
orbitals (Fig. 15).
It is difficult to even imagine that Nature has provided electrons with such bizarre areas of their most likely location. It is not easy to find any real images with which to compare these orbitals. Eight unusual conglomerates resembling clusters of peas and coffee beans, and all of this is topped by a spacecraft assembled from five tori of different sizes, pierced by two drop-shaped bodies. All these nine orbitals are incomprehensibly placed around one atomic nucleus, without interfering with each other. Our imagination is unable to imagine something like this, because other rules apply here - the laws of quantum mechanics. Of course, our imagination loses in competition with such reality. s Not exactly, but understandable R Let us return again to the CH 4 methane molecule shown on the right side of Fig. 1. The carbon atom, like all subsequent elements, has four orbitals at the second energy level (one s and three R). In addition, carbon has four valence electrons, two of which are located on R-carbon orbital is not occupied.
At the moment when a carbon atom forms four chemical bonds with four hydrogen atoms, all four orbitals seem to merge, forming hybrid orbitals (Fig. 16, top right), which are shaped like asymmetrical three-dimensional figure eights (a large drop and a small tail) . To indicate what hybrid orbitals are made from, they usually write -
sp 3-orbitals, i.e. obtained from one s- and three R-orbitals (how many orbitals are involved in the formation of hybrids, the same number of hybrid orbitals are obtained).
Such pictures can be seen in all organic chemistry textbooks, and the true appearance of the hybrids is shown in Fig. 17. In order to more clearly show their shape, the hybrid orbitals were depicted at some distance from each other (Fig. 17, left). To see the whole picture in reality, these orbitals must be combined in space so that the four white dots coincide (this is where the carbon nucleus is located). The result is shown in Fig. 17, right.
Further, these four orbitals, directed towards the vertices of an imaginary tetrahedron, overlap with the spherical orbitals of four hydrogen atoms, which corresponds to the formation of four chemical bonds (see Fig. 1). This is where purely graphical difficulties arise - if you bring four spheres close to a figure consisting of “sticking together” spherical volumes (see Fig. 17, right), then you will not be able to make out anything in such a picture. Everything looks much clearer if the hybrid orbitals are deliberately stretched (see Fig. 16). Thus, the true form of orbitals is constantly distorted for the sake of clarity, and it is difficult to object to anything here, however, for lovers of the accuracy of Fig. 17 will help you mentally imagine what everything really looks like.
Orbitals - a source of creativity
If chemists usually do not go further in their reasoning d-orbitals, f- And orbitals follow nine they are less interested in orbitals, then people of other professions quickly paid attention to the last two groups, primarily because of their extraordinary architectural attractiveness.
Artists creating samples of furniture, shoes, and household appliances could not ignore these popular images. Now orbitals can also be seen on city emblems, d-orbitals are featured on one of the pacifist emblems, and R-orbitals have long served as a model in the manufacture of hourglasses (Fig. 19).
Orbital design looks especially good in architecture, where it adorns bridge supports and television towers. By the way, the forms orbitals follow nine the orbitals surprisingly accurately correspond to the ideal parameters of the relay antennas (Fig. 20).
This entire artistic movement, called orbital design, additionally attracts buyers and customers with the alluring sonority of the new term.
What is serious and what is with a smile?
The appearance of all the orbitals shown, despite their somewhat fantastic nature, is the result of precise calculations and is completely true. How serious is the direction in artistic creativity with the general name “orbital design”, we give the readers the opportunity to decide for themselves. In chemistry, it is quite common to find a combination of serious and humorous topics presented together. In previous years, the April issues of the Khimiya newspaper regularly published various materials of this kind. From these publications one could learn: how to predict fate using the periodic table, what periodic tables exist for pharmacists, gourmets and lovers of various drinks, whether it is possible to use polymer chemistry to make the procedure of taking medications extremely pleasant, how to become famous in chemistry, features of live communication between chemists and much more.